Global Supply Chain Solutions, Regulatory Compliance Expertise Lead to Increased Revenue | Life Sciences
2/24/2025
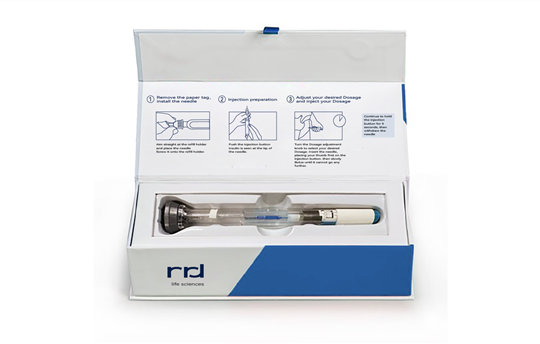
RRD Asia's end-to-end global supply chain support helps leading pharma company deploy product demonstration kits, undergo global expansion
CHALLENGE
A leading global pharmaceutical company sought external support in developing and deploying product demonstration kits. The company faced significant challenges throughout the project, including difficulties maintaining high-quality standards and managing complex regulations. Additional challenges included:
- Fragmented supply chain: The project involved numerous suppliers across the globe, requiring close coordination and alignment to ensure consistent production and on-time delivery.
- Technological challenges: A robust tech stack was needed to manage production files, global order entry, production, and fulfillment — and provide end-to-end visibility of key supply chain KPIs.
- Market expansions: Initially, the project was deployed in the U.S. and in European markets, but the recent expansion into new regions such as the Middle East, South America, and Oceania introduced additional complexities.
- Regulatory complexity: As the kits were destined for multiple international markets, each necessitated country-specific manuals and localization of box and language content. They also required approval from each affiliate’s regulatory and legal bodies.
Ultimately, the company sought support in medical product packaging design, production, final kit assembly, and global fulfillment from a trusted partner.
SOLUTION
The company turned to RRD Asia for their expertise in integrated services and global supply chain management.
RRD stood out from other suppliers through a combination of their expansive global footprint, reliability, robust customer service, and ability to support medical and demonstrator device solutions. RRD’s comprehensive solution included:
- End-to-end support: Previously, the company’s demos, boxes, and labels were produced in China, but warehousing, kitting, and fulfillment were taking place in the U.S. RRD deployed an “Asia-direct” end-to-end supply chain solution, streamlining the process, and enhancing efficiency and cost savings. This included:
- Designing and producing print and packaging components
- Sourcing non-printed items
- Managing customer-consigned stock
- Packout and global fulfillment
Automating pack out: RRD Asia developed an automated machine specifically designed to label core demonstration syringe devices in-house. This innovation guarantees accuracy and consistency across all items, significantly reducing errors, improving productivity, and reducing overall costs.
- Regulatory changes: RRD Asia quickly adapted to changes in regulatory documentation requirements which was crucial for global expansions. RRD modified documents swiftly, facilitating urgent deliveries within a week of approval — including the entire set of packaging materials.
- RRD tech stack: The RRD Life Sciences Portal was deployed to receive orders, manage files, and provide status updates, giving visibility to key KPIs. Throughout the entire project, RRD maintained close oversight and real-time management, ensuring smooth execution. Despite the complexity of managing over 100 SKUs and 600 items with limited instructions, RRD excelled in file management and achieved seamless project delivery.
RESULTS
RRD’s specialized assembly capabilities, expertise in handling logistics, and regulatory adherence in diverse markets helped the company achieve a notable increase in sales revenue. Additional benefits include:
- Reducing overall supply chain complexity and cost: RRD Asia redesigned the company’s supply chain, relocating all functions — packaging production, automated device labeling, final packout, and global fulfillment — to a single site in Asia.
- Expanded capabilities and reach: A dedicated medical assembly capability — including a cleanroom and ISO 13485 certifications — was implemented in Asia. In addition, RRD provided expertise in managing logistics and regulatory requirements for new markets in the Middle East, South America, and Oceania.
- Efficient project management: The RRD team managed the complexities associated with FDA approvals, fostering effective communication among various stakeholders. RRD also efficiently coordinated production and delivery schedules across multiple countries.
- Consistent business growth: RRD’s collaborative, consultative support is highly valued and has led to a consistent rise in business.